Preterm nutrition products
Benefits of an Exclusive Human Milk Diet (EHMD)
Prolacta Bioscience, an industry leader at the forefront of advancing the science of human milk, is proud to support hospitals and clinicians with an evidence-based resource to help address the nutritional risks facing low birth weight premature infants. Prolacta’s Exclusive Human Milk Diet ProtocolTM † provides flexible and clinically proven guidance on using Prolacta’s human milk-based nutritional products † in the neonatal intensive care unit (NICU).
Prolacta’s human milk-based products have been evaluated in more than 30 clinical studies with more than 6,5000 premature infants.1
Our products, as part of an exclusive human milk diet (EHMD), have been shown in clinical studies to:
Our products
Prolacta’s human milk–based nutritional products contain a wide range of human milk oligosaccharides (HMOs), special sugars abundantly found in human milk.9 HMOs promote the development and maturation of the newborn immune system and provide a supplementary source of sialic acid critical for brain development. 10,11
Prolacta’s Humavant+ fortifier
Prolacta’s Humavant+ fortifier is the first commercially available human milk fortifier made from 100% human milk.

HumavantTM CR Human Milk Caloric Fortifier
Human milk caloric fortifier is ideal for neonatal infants receiving low caloric content. Data show that 65% of the time, term mother’s own milk (MOM) is less than 20 Cal/fl oz.12 Humavant CR human milk caloric fortifier can meet the need for additional calories.
- Intended for use with mother’s own milk (MOM) or donor breastmilk (DM) to increase lipids and achieve adequate growth
- Formulated to deliver at least 2.6 Cal/mL
- Available frozen in bottles containing 10 mL of product
(4 bottles per unit carton, with 48-hour expiration) - Includes simplified preparation instructions
- Easy to use and measure

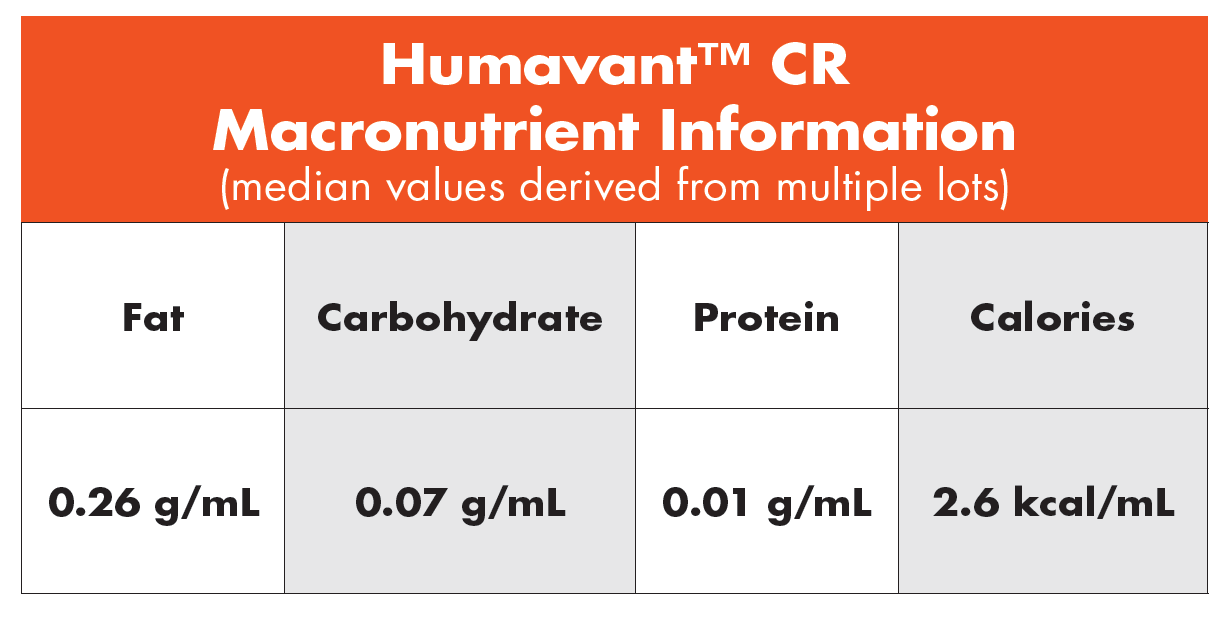
Humavant CR human milk caloric fortifier is the only completely human solution created to add calories to mother’s own milk (MOM) or donor breastmilk (DM) without substantially increasing volume and without introducing a non-human milk-based nutritional product.
A randomised clinical trial found that premature infants who received an exclusive human milk diet (EHMD) with Humavant CR fortifier had superior length and weight velocity compared to infants who received an exclusive human milk diet (EHMD) without Humavant CR fortifier.13

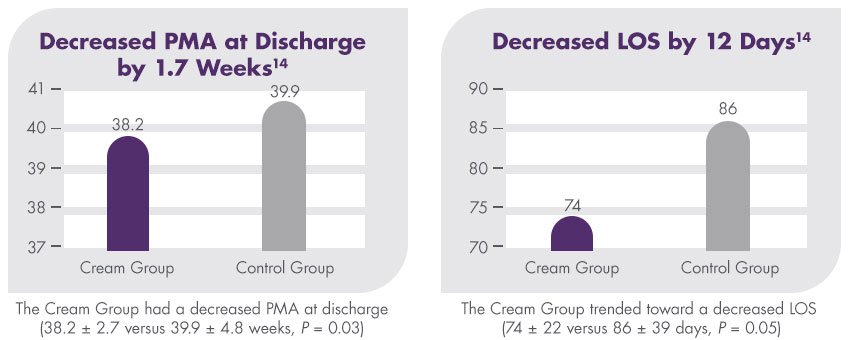
This study is a subset analysis of data originally published in 2014 by Hair et al in The Journal of Pediatrics.
Clinical Studies and Publications:
- Randomised Trial of Human Milk Cream as a Supplement to Standard Fortification of an Exclusive Human Milk-Based Diet in Infants 750-1250 g Birth Weight
- Premature Infants 750–1,250g Birth Weight Supplemented with a Novel Human Milk-Derived Cream Are Discharged Sooner
- Fortifier and Cream Improve Fat Delivery in Continuous Enteral Infant Feeding of Breast Milk
- Macronutrient analysis of a nationwide sample of donor breast milk
Product information downloads
Humavant® RTF
Humavant® RTF (Ready-To-Feed) offers Special Care Baby Units (NICU)s superior solutions for their extremely premature infants when mother’s own milk is not available.
Human Milk-Based Premature Infant Formula
- Provides an easy, convenient, labour saving way to provide 100% human milk-based diet when mother’s own milk is unavailable
- A more cost-effective way to provide an exclusive human milk diet comprised of HumavantTM HM and Prolacta’s HumavantTM+ fortifier
- Reduces the chance of mixing errors and saves time
- Shorter duration of TPN for infants fed an Exclusive Human Milk-based Diet vs. cow milk-based preterm formula4
- Significantly fewer surgical Necrotising enterocolitis (NEC) cases for infants fed an Exclusive Human Milk-based Diet vs. cow milk-based preterm formula4

Overcoming complications
Prolacta’s products, when used as part of an exclusive human milk diet (EHMD), have been shown to reduce the incidence of clinical complications.
In a multicentre, retrospective cohort study with 1587 patients, the outcomes of extremely premature infants (<1250 g birth weight) who received a diet including cow milk–based products were compared with infants who received an Exclusive Human Milk Diet (EHMD). The incidence of mortality, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and late-onset sepsis were all reduced with an EHMD.18
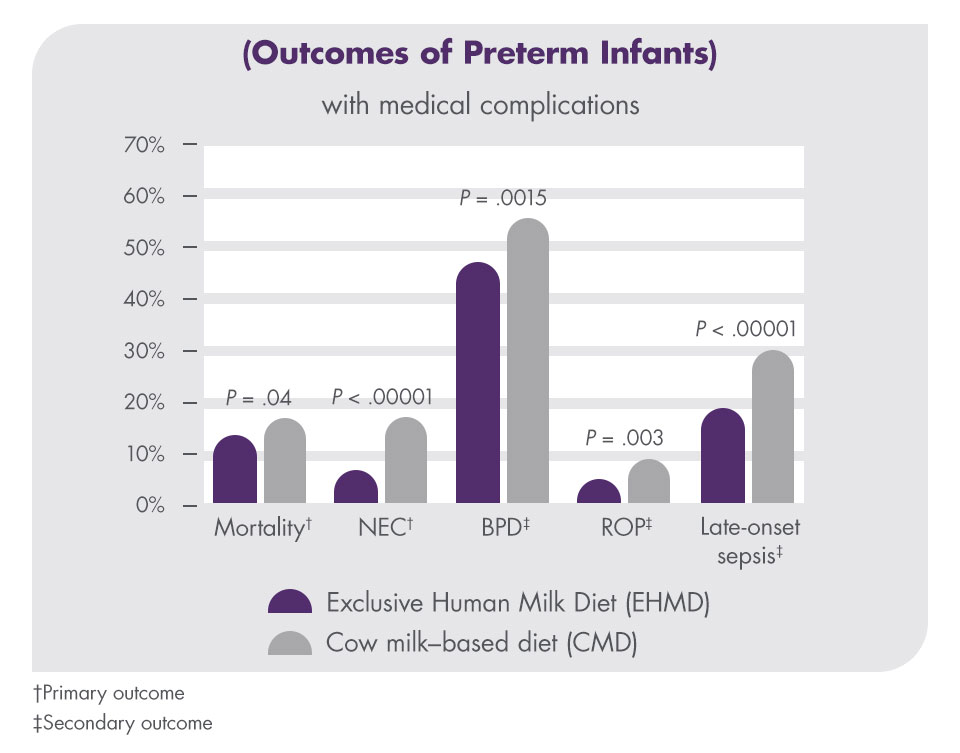
Improving outcomes with Prolacta's exclusive human milk diet (EHMD)
- Decreasing feeding intolerance – Prolacta's exclusive human milk diet (EHMD) was associated with a lower incidence of feeding intolerance and shorter time to full feeds compared to a diet containing cow milk-based products2
- Supporting adequate growth – An exclusive human milk diet (EHMD) feeding protocol for infants with a birthweight of ≤1250 g that enables early and rapid fortification advancement is associated with weight, length and head circumference gains meeting targeted standards, and with a low rate of extrauterine growth restriction6,7,19
- Turning to nutrition to address the complications of prematurity – Today, more and more hospitals are turning to Prolacta’s fortifiers that are clinically proven as part of an exclusive milk diet (EHMD); this nutritional solution is shown in a growing body of evidence to reduce the most serious complications of prematurity3,4,5,18
Reducing hospital costs with an Exclusive Human Milk Diet (EHMD)
Very low birth weight babies are at risk for prematurity-related morbidities and interventions, such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), late-onset sepsis, and necrotising enterocolitis (NEC). The incremental cost of these morbidities and interventions can substantially increase the cost of Special Care Baby Unit (NICU) hospitalisation.

